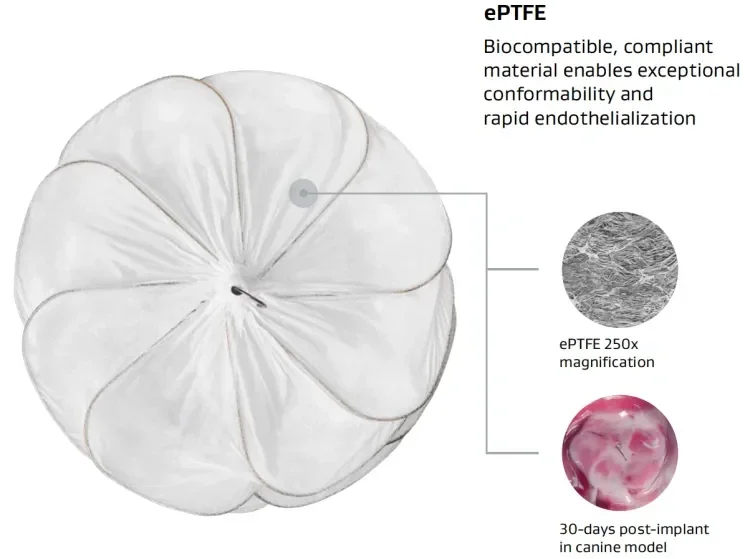
GORE® CARDIOFORM ASD Occluder
Advanced materials delivering exceptional conformability†, §, II, 1
- Developed by a company with 60 years of materials science experience
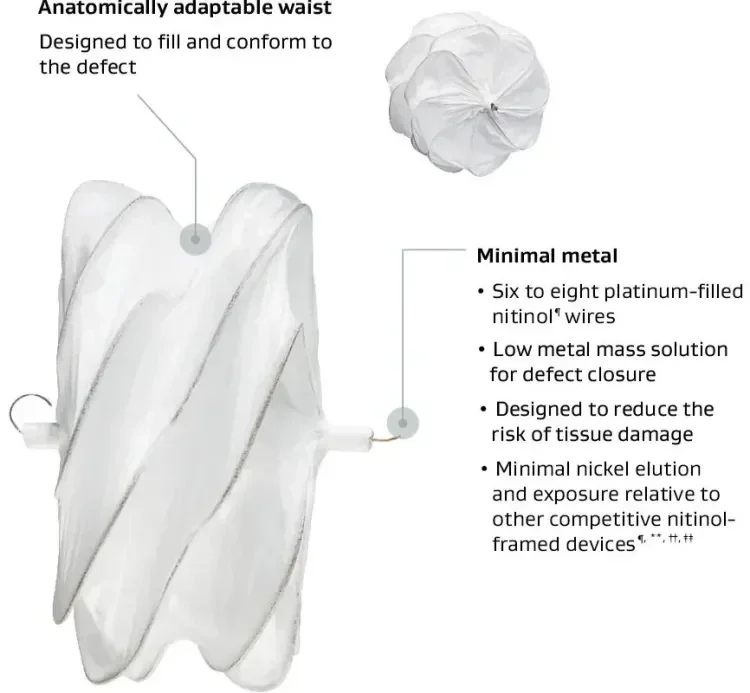
- Engineered to conform to a broad range of ASD anatomies§,1,2
- No minimum retro-aortic rim requirements2
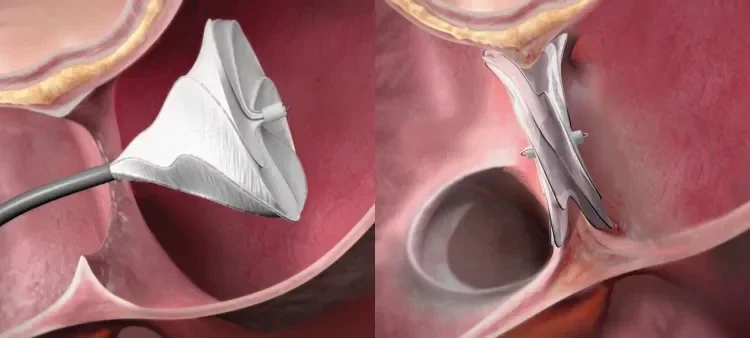
See deficient rim ASD closure cases
Trusted deployment††, 2
- Straightforward delivery with the ability to retrieve and reposition††, 2
- Pre-assembled occluder and delivery system2 designed to reduce device preparation time
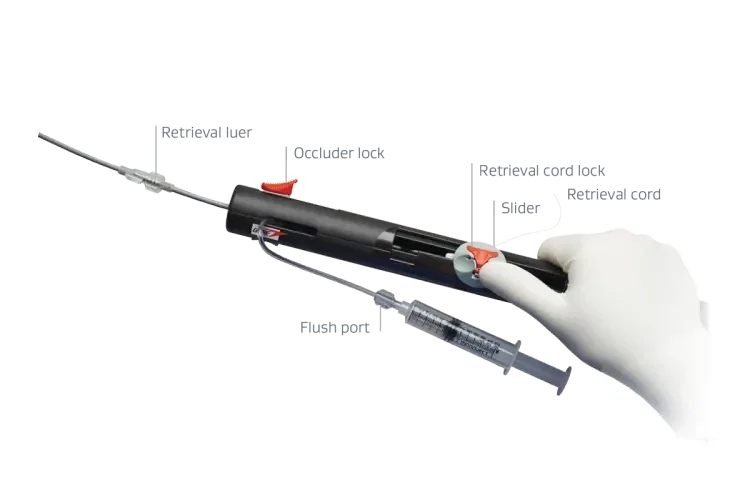
The built-in retrieval cord allows for tension-free assessment and post-lock retrieval, if needed.2
Watch how GORE® CARDIOFORM ASD Occluder is deployed
1-2-3 Deployment sequence††
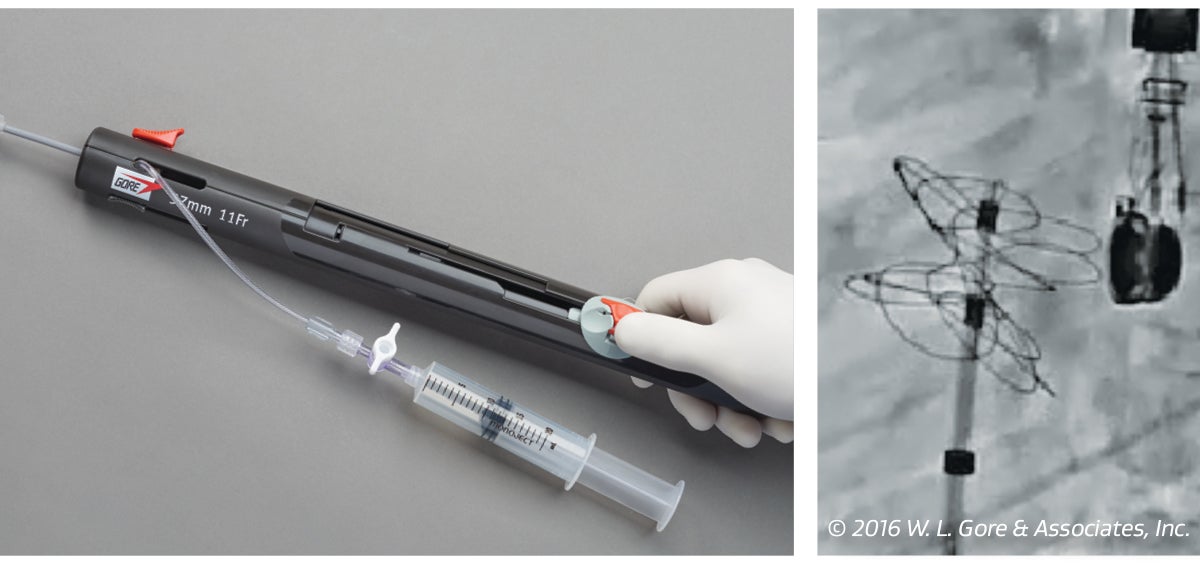
1. Deploy
Handle design with slider enables accurate deployment with the ability to reposition.
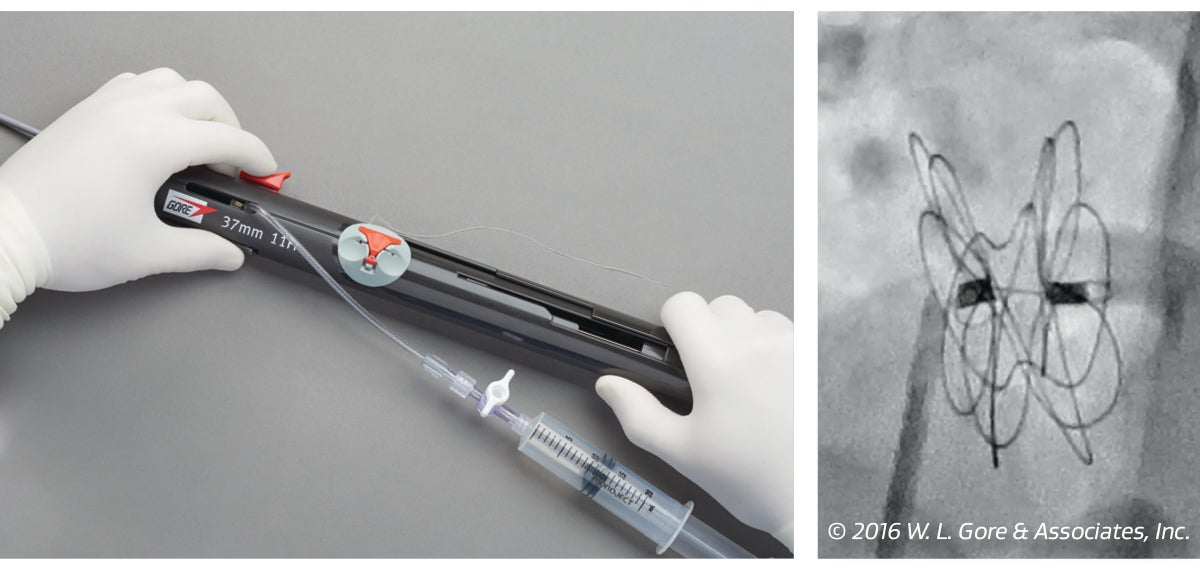
2. Lock
Simple-to-use locking mechanism. Tension-free assessment post-lock where the occluder remains tethered to the delivery system.
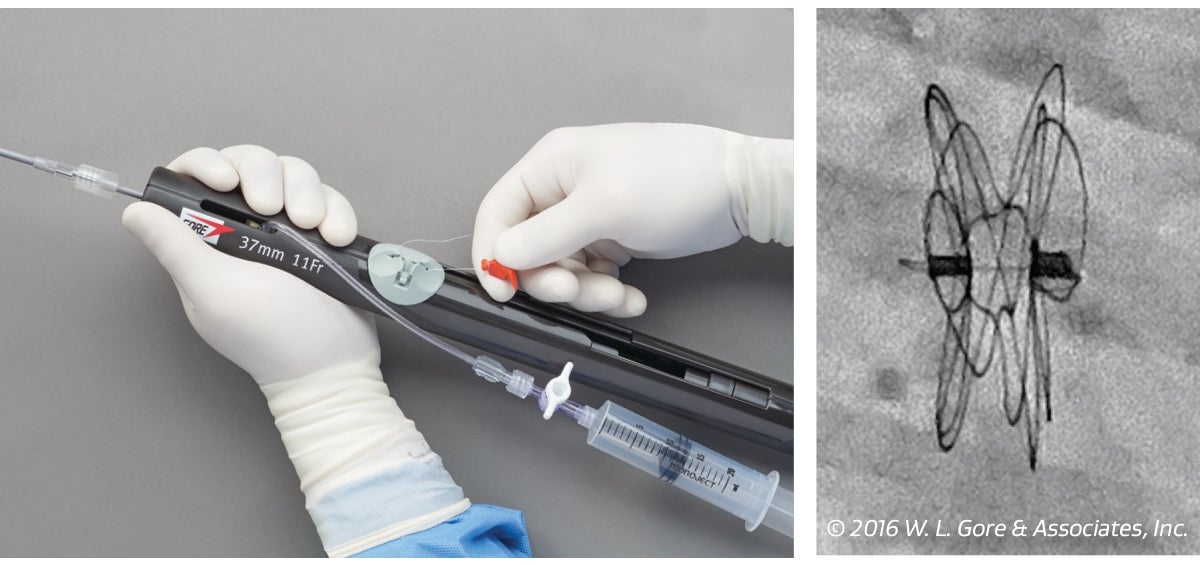
3. Release
Pull the retrieval cord until completely removed to release the device from the delivery system.
* Deficient retro-aortic rim was defined as a retro-aortic rim measuring less than or equal to 5 mm on any view on echocardiogram.
† Closure success defined as completely occluded or clinically insignificant shunt as determined by the Echo Core Lab at the six-month evaluation among subjects with technical success.2
‡ Reported incidence rate of device-related cardiac erosions for GORE® CARDIOFORM ASD Occluder calculated using data from CATSWeb® Product Surveillance Tracking System [PSTS] and SmartSolve®. Data on file. March 1, 2015 – May 31, 2023; W. L. Gore & Associates Inc.; Flagstaff, AZ.
§ All ASD anatomies were eligible for inclusion into the ASSURED Clinical Study within indicated sizing parameters of the Instructions for Use.
II 100% closure success rate across ASD anatomies at six months.†,§,1
¶ Nickel titanium.
** Patients allergic to nickel may suffer an allergic reaction to the GORE® CARDIOFORM ASD Occluder. Certain allergic reactions can be serious; patients should be instructed to notify their physicians immediately if they suspect they are experiencing an allergic reaction such as difficulty in breathing or inflammation of the face or throat. Some patients may also develop an allergy to nickel if this device is implanted.
†† Refer to Instructions for Use at eifu.goremedical.com for a complete description of all applicable indications, warnings, precautions and contraindications for the markets where this product is available. Rx only
‡‡ Data on file. W. L. Gore & Associates, Inc.; Flagstaff, AZ.
1. Sommer RJ, Love BA, Paolillo JA, et al.; ASSURED Investigators. ASSURED clinical study: new GORE® CARDIOFORM ASD Occluder for transcatheter closure of atrial septal defect. Catheterization & Cardiovascular Interventions 2020;95(7):1285-1295.
2. GORE® CARDIOFORM ASD Occluder [Instructions for Use]. Flagstaff, AZ.: W. L. Gore & Associates, Inc; 2023. MD190349.

INDICATIONS FOR USE IN THE U.S.: The GORE® CARDIOFORM ASD Occluder is a permanently implanted device indicated for the percutaneous, transcatheter closure of ostium secundum atrial septal defects (ASDs).
CONTRAINDICATIONS: The GORE® CARDIOFORM ASD Occluder is contraindicated for use in patients: Unable to take anti-platelet or anticoagulant medications such as aspirin, heparin or warfarin; with anatomy where the GORE® CARDIOFORM ASD Occluder size or position would interfere with other intracardiac or intravascular structures, such as cardiac valves or pulmonary veins; with active endocarditis, or other infections producing bacteremia, or patients with known sepsis within one month of planned implantation, or any other infection that cannot be treated successfully prior to device placement; with known intracardiac thrombi.
Refer to Instructions for Use at eifu.goremedical.com for a complete description of all applicable indications, warnings, precautions and contraindications for the markets where this product is available. RXOnly
CATSWeb is a trademark of AssurX, Inc. SMARTSOLVE is a trademark of Ethos Technologies Inc.
23834775-EN